What does the term nucleophile mean?
A nucleophile is an electron-rich chemical species that form covalent bonds with electrophiles. This includes an atom or molecule with one or more lone electron pairs that can be donated or shared to form a single bond. This Nucleophile can either be a neutral or negatively charged chemical species.
Before diving into understanding nucleophilic substitution reaction and the drawing SN1 and SN2 comparison, let us briefly understand the basics.
What does the term Nucleophilic Functional Groups mean?
Below are the examples of Nucleophiles –
H2O NH3 OH– Cl–
Nucleophilic functional groups, on the other hand, are those groups of chemical species which have electron-rich atoms. They can donate an electrons pair to form a new covalent bond with other electrophiles. The most common and regularly studied nucleophilic atoms in organic chemistry include nitrogen, oxygen, and sulphur. Also, the most common nucleophilic functional groups include alcohols, phenols, thiols, water, amines, and carboxylates as well.
Moreover, carbons can also be nucleophiles. We have seen the synthesis of larger molecules like in fatty acids and DNA with carbon-carbon bonds. And as such, enolate ions are some of the examples of carbon nucleophiles seen in biochemical reactions. Also, the halide and azide (N3-) anions and the cyanide ion (CN-) are examples of nucleophiles widely used in laboratory experiments.
When we discuss nucleophiles, the most important concept that we need to understand is the electron-richness that a nucleophile constitutes. This characteristic makes nucleophilic molecules basic. Thus, nucleophiles can be bases, or in other words, bases can be nucleophiles as well.
Nucleophilic Substitution Reaction Mechanism?
A nucleophilic substitution reaction is a type of reaction in which an electron-rich nucleophile replaces the leaving group. It selectively binds the positive ions or joins a partially positive charge atom or a group of atoms to make the bond.
In a nucleophilic substitution reaction, one atom or functional group substitutes another, forming a covalent bond. The removed group of atoms is called a ‘leaving group’ while the replacing atom or group of atoms is called the ‘nucleophile’.
These nucleophilic substitution reactions cause an imbalance in the electrons that exist between the electrophile and leaving group. Also, it occurs mainly because it has a covalent bond. The electronegative leaving group pulls the electron present, and the electrophile becomes partially positively charged.
For instance, a carbon atom being an electrophile, need to have some number of electrons while others with high electron molecules will be attracted to this carbon atom.
Some of the common electrophiles are the halides since the partially charged Br–, Cl–, and I– are leaving groups.
Nucleophile and Leaving Group Difference
At least one lone pair of electrons is required to be qualified to be called a nucleophile. Also, very often, these nucleophiles are negatively charged since the negative polarity makes it more reactive. You can find out some of the examples of commonly used nucleophiles that you must have learned in your chemistry class lectures.
To have a nucleophilic substitution reaction, having a nucleophile and a carbon atom with a negatively charged electron is not enough. You also need to have a leaving group as well. For the leaving group to be removed by the nucleophile, it needs to have the characteristics of a weak base, i.e. able to handle the negative charge.
Also, a good leaving group will usually be a conjugate base of a strong acid molecule. So, if the conjugate base or the leaving group is more stable, the acid becomes stronger.
What are Nucleophilic Substitution Reactions?
Nucleophilic substitution reactions are a group of chemical reactions that involve the interaction of an electrophile with that of a nucleophile. The nucleophile – an electron-rich species attacks the electrophile, which is electron-deficient. As the electrophile contains an atom or a group of atoms called the ‘leaving group’, it ultimately detaches itself from the electrophile. This particular atom or group is the negatively charged species mentioned earlier.
Types of Nucleophilic Substitution Reactions?
SN1 and SN2 Comparision –
There are two types of Nucleophilic Substitution Reactions. These are called SN1 and SN2 reactions. Each of these reaction types has its functioning mechanism.
The ‘S’ in SN2 stands for substitution, while the ‘N’ stands for nucleophilic. Also, 1 and 2 in SN1 and SN2 refers to the molecularity of the reaction type. Furthermore, the number 1 is assigned when the reaction time depends on the electrophile; if not, the number 2 is assigned for electrophile and nucleophile chemical species.
Moreover, while the SN2 reaction mechanism happens in a single step, the SN1 reaction typically occurs in two steps. However, the numbers 1 and 2 do not reflect the number of steps that takes place in each reaction mechanism.
So, to remove the confusion, the 1 and 2 in SN1 and SN2 generally refers to the kinetics in the respective reactions. Thus, the SN2 reaction and its steps depend on nucleophilic and electrophilic concentration, whereas the SN1 reaction only depends on the electrophile concentration.
Let us discuss more on these two main types of substitution reactions. In the first reaction type, the nucleophilic reaction and the release of the leaving group happen simultaneously. When such a reaction happens simultaneously, it is called a concerted reaction mechanism. In other words, it is called the SN2 reaction mechanism type.
In the second type of reaction, the release of the leaving group happens much before the reaction of the nucleophile. This is called a stepwise mechanism since the processes happen in steps. This type of reaction is called the SN1 reaction type.
The SN2 Reaction Mechanism
When it comes to an SN2 reaction, a nucleophile targets the atom of the electrophile. The covalent bond is formed between the nucleophile and carbon atom simultaneously when the bond between the carbon atom and leaving group breaks apart. Or in other words, ‘Nucleophile-Carbon’ bond formation and breaking of the ‘Carbon-Leaving Group’ bond happen simultaneously. In this transitionary state, the carbon atom is partially attached to both of them.
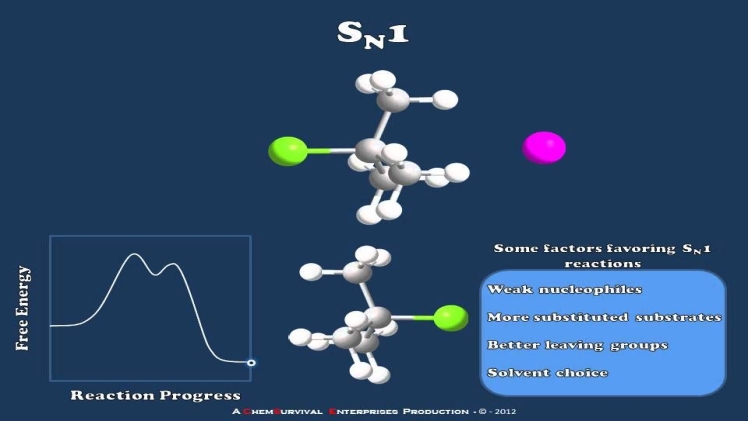
The SN1 Reaction Mechanism
The SN1 reaction mechanism involves a nucleophilic substitution reaction that proceeds in two simple steps. Firstly, the existing bond between the carbon atom and the leaving group breaks apart to produce a carbocation. This is an anionic leaving group that is formed in most cases.
Secondly, the carbocation reacts immediately with the nucleophile and forms a second chemical species. The carbocation formation process is slow or, in other words, a rate-determining step. The next step i.e. the formation of a bond between the carbocation and the nucleophile, occurs immediately. Because of this slow process, it involves only the substrate, and as a result, the reaction is unimolecular.
Conclusion
Chemistry is one of the most important and challenging subjects, yet you can score good marks in your exams. Focus on your theoretical knowledge while being able to solve the relevant numerical problems is the key. Learning the formulas and solving numerical regularly will increase your overall speed in the exam.